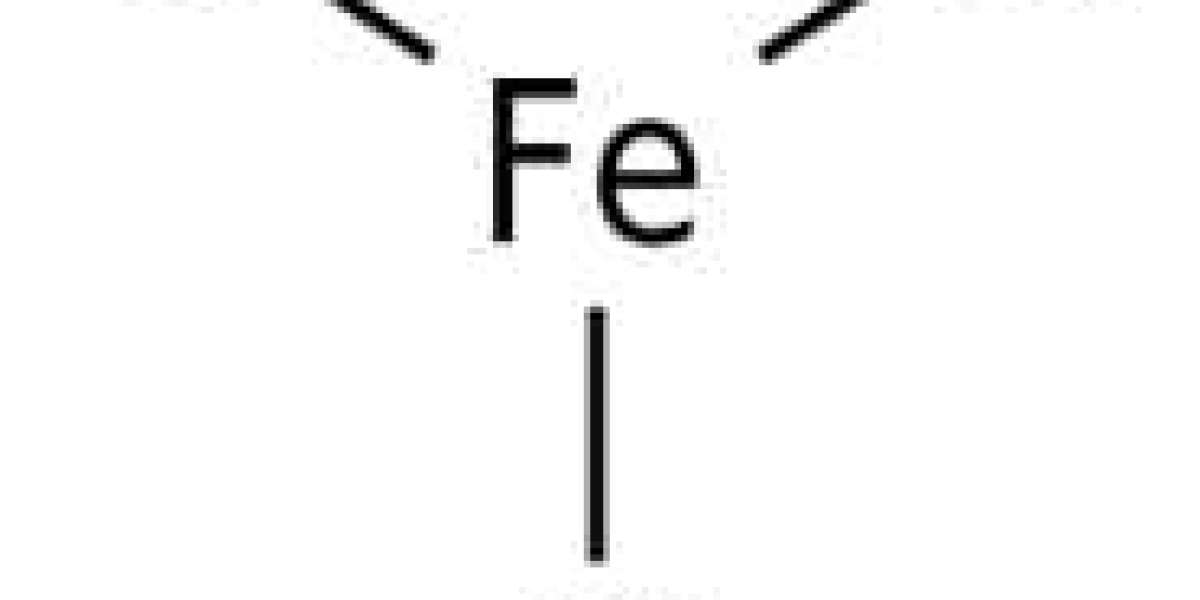Ferric chloride is an inorganic compound with the chemical formula FeCl3. Also known as ferric chloride, it is a common iron compound in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: in reflected light the crystals appear dark green, but in transmitted light they appear purple.
Anhydrous ferric chloride has a BiI3 structure with octahedral Fe(III) centers interconnected by dicoordinated chloride ligands. [3]
Iron(III) chloride has a relatively low melting point and a boiling point of about 315 °C. The vapor consists of dimer Fe2Cl6 (like aluminum chloride), which increasingly dissociates at higher temperatures into monomeric FeCl3 (with D3h point group molecular symmetry), competing with its reversible decomposition to produce iron chloride (II ) and chlorine gas. [
In addition to anhydrous, ferric chloride forms four hydrates. All forms of iron(III) chloride have two or more chlorides as ligands, and the three hydrates have [FeCl4]−. [9]
Dihydrate: The structural formula of FeCl3 2H2O is trans-[FeCl2(H2O)4][FeCl4].
The structural formula of FeCl3·2.5H2O is cis-[FeCl2(H2O)4][FeCl4]·H2O.
The structural formula of FeCl3·3.5H2O is cis-[FeCl2(H2O)4][FeCl4]·3H2O.
Hexahydrate: FeCl3 6H2O, the structural formula is trans-[FeCl2(H2O)4]Cl 2H2O[10]
In contrast to the pale pink solution of [Fe(H2O)6]3+, the aqueous solution of ferric chloride is yellow. Based on spectroscopic measurements, the dominant species in aqueous ferric chloride were the octahedral complex [FeCl2(H2O)4]+ (stereochemistry not specified) and the tetrahedral [FeCl4]−. [9]
Anhydrous iron(III) chloride can be prepared by treating iron with chlorine:[11]
2 Fe + 3 Cl2 → 2 FeCl3
Iron(III) chloride solutions are produced industrially from iron and ores by a closed-loop process.
Heating ferric(III) chloride hydrate does not produce anhydrous ferric chloride. Instead, the solid decomposes into hydrochloric acid and ferric oxychloride. Iron(III) chloride hydrate can be converted to the anhydrous form by treatment with thionyl chloride. [12] Likewise, dehydration can be performed with trimethylchlorosilane: [13]