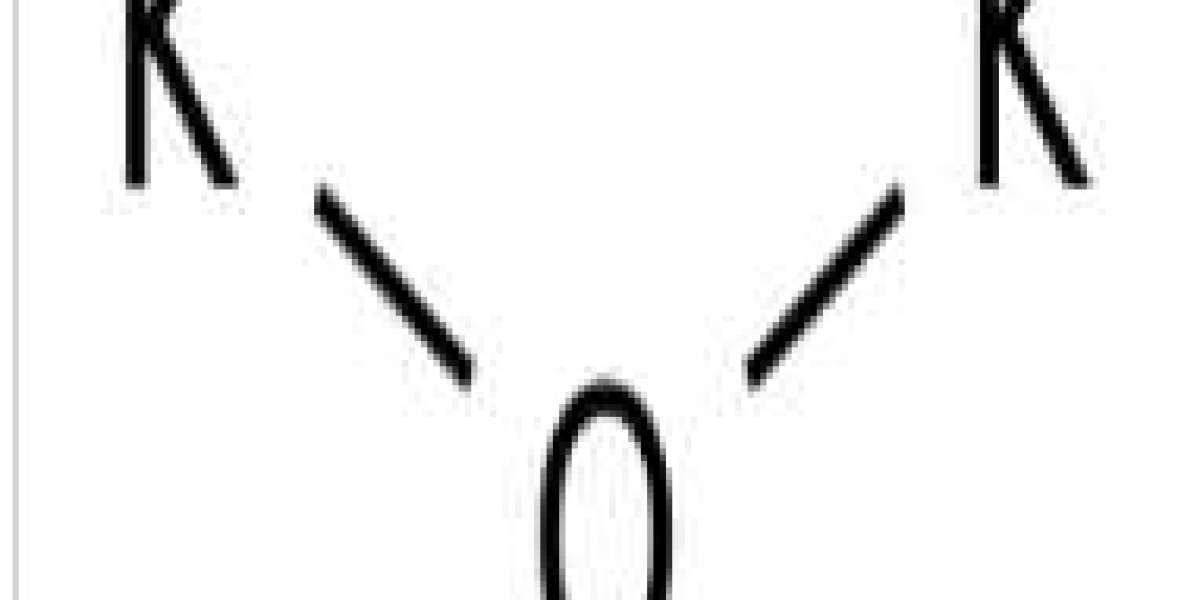
Potassium oxide (K2O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest potassium oxide. It is a highly reactive compound that is rarely encountered.
Potassium oxide is an ionic compound formed by combining potassium and oxygen. It has the chemical formula K2O. Can't find free potassium because it's too reactive. It has a valence of +1 and readily combines with an oxygen atom to form K2O. When potassium is oxidized, the oxide K2O is obtained as a gray crystalline substance; potassium burns in excess oxygen to form potassium oxide. Potassium oxide is a strongly corrosive base when dissolved in water.
Potassium Oxide – Uses of K2O
Potassium oxide or "pure potash" expressed as K2O has been designated as a commercial standard or unit.
It is used in agriculture as a fertilizer, but is also used to make glass and soap, and in small quantities for medicinal purposes.
For the treatment of fungal granulomatosis and infections associated with zygomycetes.
It has been used for over 100 years to treat actinomycosis and actinomycosis in cattle; it is also used to treat sporotrichosis and botrytis.
What is potassium oxide used for?
It is widely used as fertilizer in agriculture. Potassium oxide is also used to make soap and to make glass. Certain medical procedures are also known to involve potassium oxide.
Is potassium oxide acidic or basic?
Potassium oxide is a basic oxide. Other important examples of basic oxides include FeO (iron oxide) and CaO (calcium oxide).