Anhydrous chromium(III) chloride can be prepared by direct chlorination of chromium metal or indirectly by carbothermal chlorination of chromium(III) oxide at 650–800 °C.
Chromium(III) chloride is used as a precursor for many organochromium compounds, such as bis(phenyl)chromium, an analog of ferrocene:
Chromium(III) chloride is also used as a Lewis acid in organic reactions, such as catalyzing the nitroso-Diels-Alder reaction.
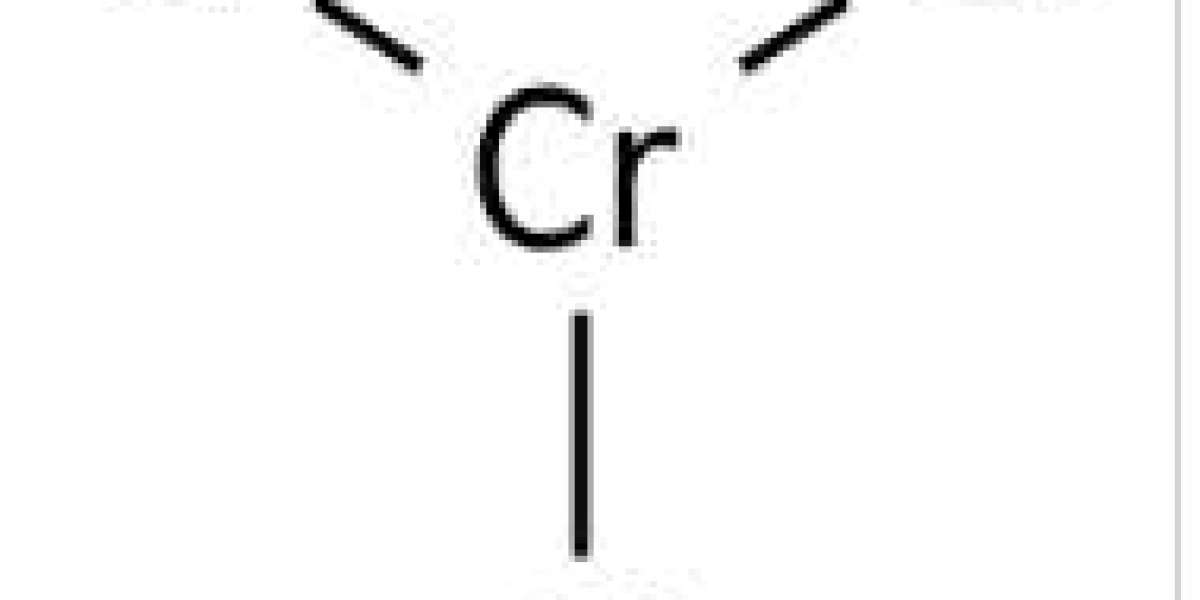
Chromium(3+) trichloride is chromium chloride with the chromium cation in the +3 oxidation state. It acts as a Lewis acid and a sensitizer.
Chromium Chloride for Injection is a sterile, pyrogen-free solution intended for use as an additive to total parenteral nutrition (TPN) solutions.
Further studies of chromium chloride supplementation showed that estradiol + chromium chloride inhibited the secretion of the pro-inflammatory cytokine interleukin (IL)-6 and oxidative stress in monocytes when exposed to high glucose.
Numerous studies have been conducted to evaluate the safety of trivalent chromium. In the comparative assessment, the toxicity of chromium picolinate, chromium combined with niacin and chromium chloride was examined. Chromium picolinate showed potential mutagenicity at the hypoxanthine (guanine) phosphoribosyltransferase site in Chinese hamster ovary cells at the same physiologically relevant doses, while chromium chloride and nicotinic acid Bound chromium has not been shown to be mutagenic [69].