Exposure may occur in ambient air and during solvent use. In humans, acute (short-term) or chronic (long-term) exposure to methanol through inhalation or ingestion may cause blurred vision, headache, dizziness, and nausea. There is currently no information on the reproductive, developmental or carcinogenic effects of methanol in humans. Birth defects have been observed in the offspring of rats and mice exposed to methanol. The EPA has not classified methanol as carcinogenic.
Here's a cool fact about methanol, which occurs naturally in wood. As an alcohol, it is also known as "wood alcohol". Volcanic gases and even some fuels contain methanol. Did you know that traces of methanol are found in fruits, vegetables and juices? This is true as it is naturally found in these projects. Considering the extent to which methanol is harmful to our health, it is quite surprising to know that this chemical occurs naturally in our food. Before addressing the uses and health hazards of methanol, let's take a closer look at its chemical properties and structure.
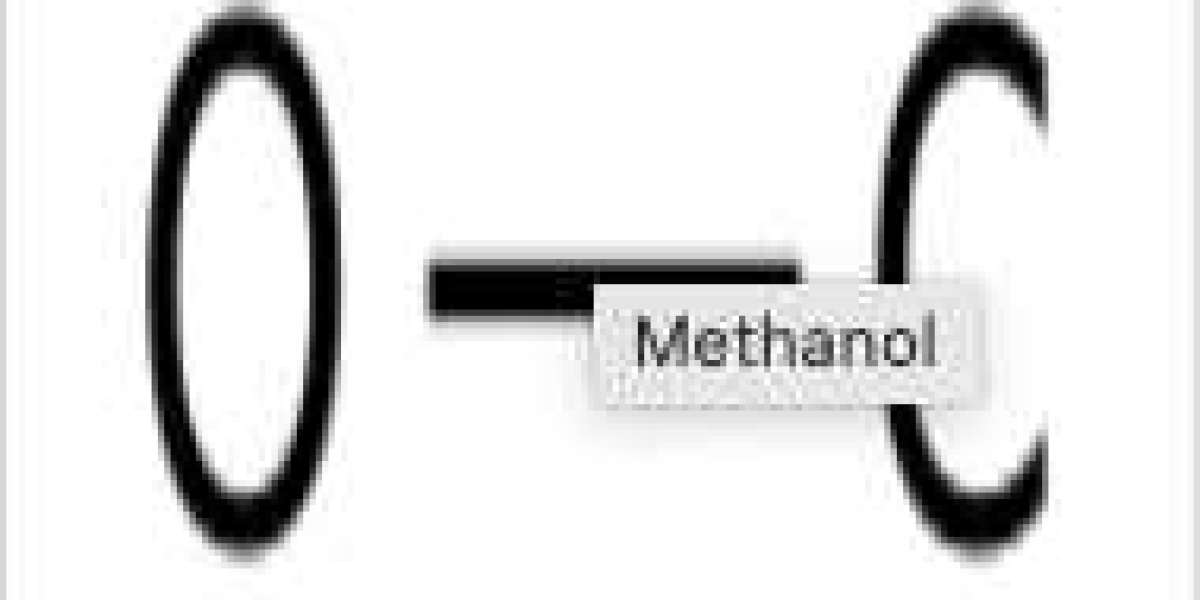
Methanol is a simple alcohol containing a methyl group (carbon and three hydrogen atoms) attached to an alcohol (OH) group. The chemical structure of methanol is shown in Figure 1. It is a clear, colorless liquid with a distinctive smell. The chemical formula for methanol is CH3OH. Its molar mass is 32.04 grams per mole. The molar mass of a chemical is a physical property (Figure 2). It is the relationship between the mass (grams) of a substance and its quantity (moles). Some safety measures are in place to protect those who may work with methanol. A major reason is concern about fire hazards. Methanol is considered highly flammable and volatile. Remember this about volatility. The more volatile a substance is, the lower its boiling point is. The boiling point of methanol is 64.6 degrees Celsius. To put that in perspective, water boils at 100 degrees Celsius. Methanol has such a low boiling point, which completely explains why methanol is so volatile!