2-Methyl-2-butene is prepared by dehydration of tert-amyl alcohol in the presence of p-toluenesulfonic acid or by distillation of catalytically cracked gasoline streams, followed by extraction with aqueous sulfuric acid at low temperatures. 2-Methyl-2-butene is a clear, colorless liquid with a petroleum-like odor. Density is less than water, insoluble in water. So float on the water. Vapor is heavier than air.
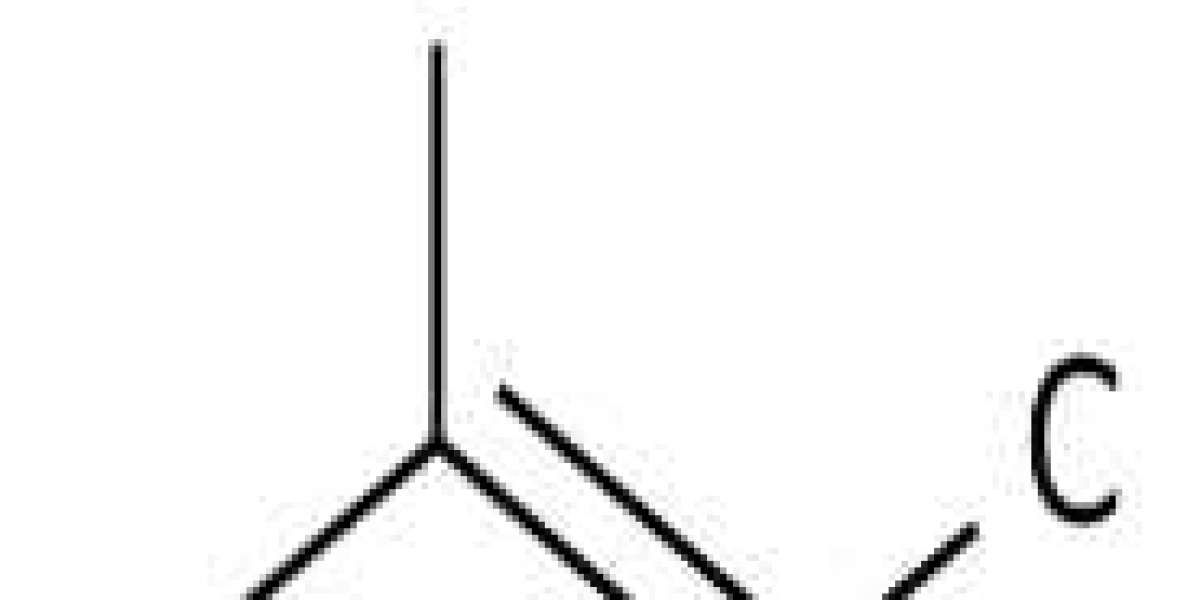
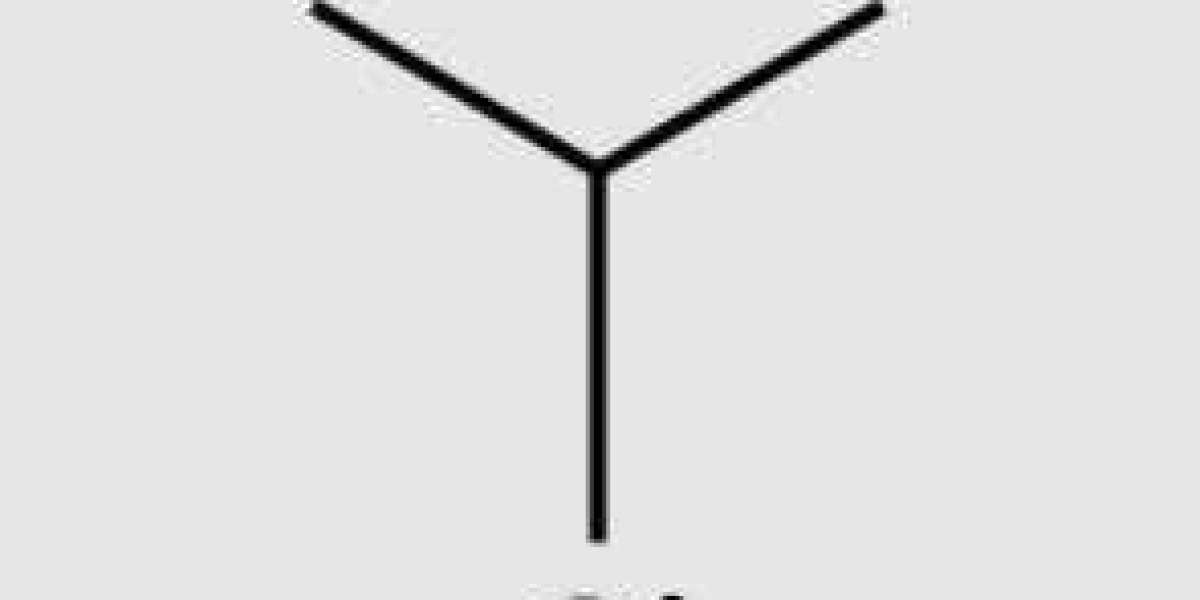
2-Methyl-2-butene is an alkene, ethylene, in which three hydrogens have been replaced by methyl groups.
2-Methyl-2-butene may react violently with strong oxidizing agents. May react exothermically with reducing agents liberating gaseous hydrogen. Exothermic polymerization reactions may occur in the presence of various catalysts (e.g. acids) or initiators.
2-Methyl-2-butene is a trisubstituted olefin. It acts as a guest to form a stable solid host-guest complex with self-assembled benzophenone diurea macrocycles. The effect of active chlorine on the photooxidation of 2-methyl-2-butene was studied. The photosensitive oxidation reaction of 2-methyl-2-butene adsorbed on the inner framework of Na-ZSM-5 molecular sieve was studied. The gas phase reaction of 2-methyl-2-butene with ozone was studied. The kinetics of the liquid-phase alkylation of 3-methylthiophene with 2-methyl-2-butene over supported phosphoric acid have been reported.
Virginie Bellière, Christophe Geantet, Michel Vrinat, Younès Ben-Taarit, Yuji Yoshimura, Alkylation of 3-methylthiophene with 2-methyl-2-butene over zeolite catalysts, Energy Fuels, 2004, Vol. 18, p. 1806-1813
- Grosjean, Gas phase reaction of ozone with 2-methyl-2-butene: Criegee diradicals to form dicarbonyls, Environmental Science Technology