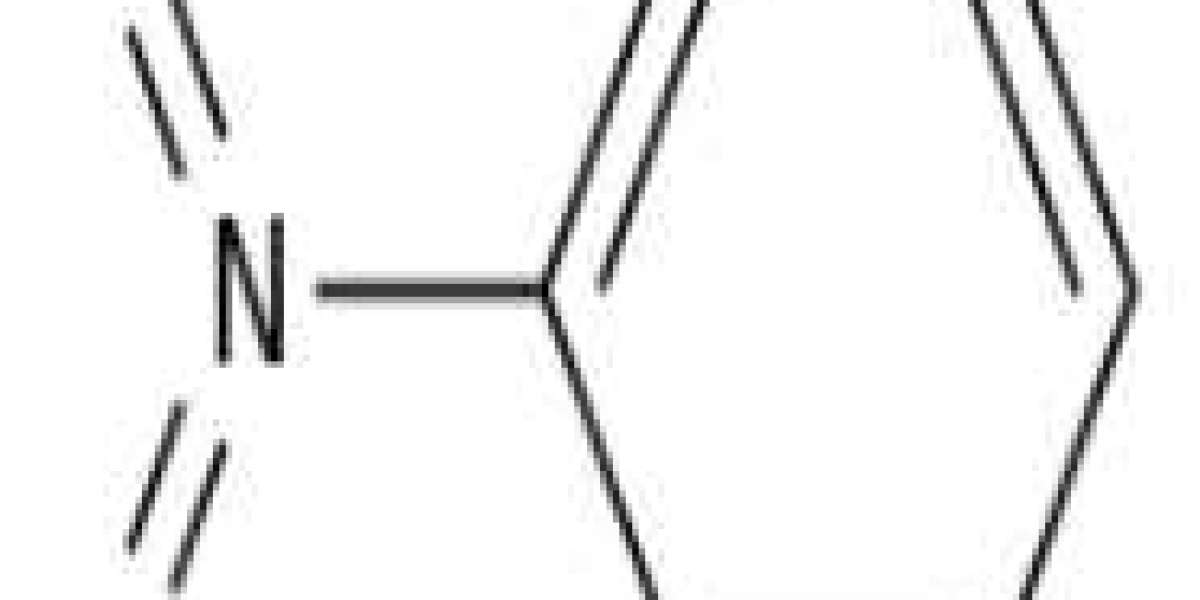
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is an insoluble, pale yellow oil with an almond-like odor. It freezes into yellow-green crystals. It is produced on a large scale from benzene as a precursor to aniline. In laboratories, it is occasionally used as a solvent, especially for electrophiles.
Nitrobenzene is produced by nitrating benzene with a mixture of concentrated sulfuric acid, water and nitric acid. This mixture is sometimes called a "mixed acid". The production of nitrobenzene is one of the most hazardous processes in the chemical industry due to the exothermic reaction (ΔH = −117 kJ/mol). [4]
Benzol.svg + nitrate vert.svg
Rightward reaction arrow with secondary product in upper right corner
Nitrobenzene.svg
In 1985, the world's nitrobenzene production capacity was about 1.7 million tons
The nitration process involves the formation of a nitronium ion (NO2+), followed by its electrophilic aromatic substitution reaction with benzene. Nitronium ions are formed by reacting nitric acid with an acidic dehydrating agent (usually sulfuric acid):
HNO3 + H+ ⇌ NO2+ + H2O
Nitrobenzene is also used to mask unpleasant odors in shoe and floor polishes, leather dressings, paint solvents, and other materials. After redistillation, nitrobenzene is used as a cheap fragrance for soaps as nitrobenzene oil. For this purpose, it has been replaced by less toxic chemicals. [5] An important commercial market for nitrobenzene is its use in the manufacture of the pain reliever paracetamol (also known as acetaminophen) (Mannsville 1991). [6] Nitrobenzene is also used in Kerr cells because of its unusually large Kerr constant. There is evidence of its use in agriculture as a plant growth/flowering stimulant.