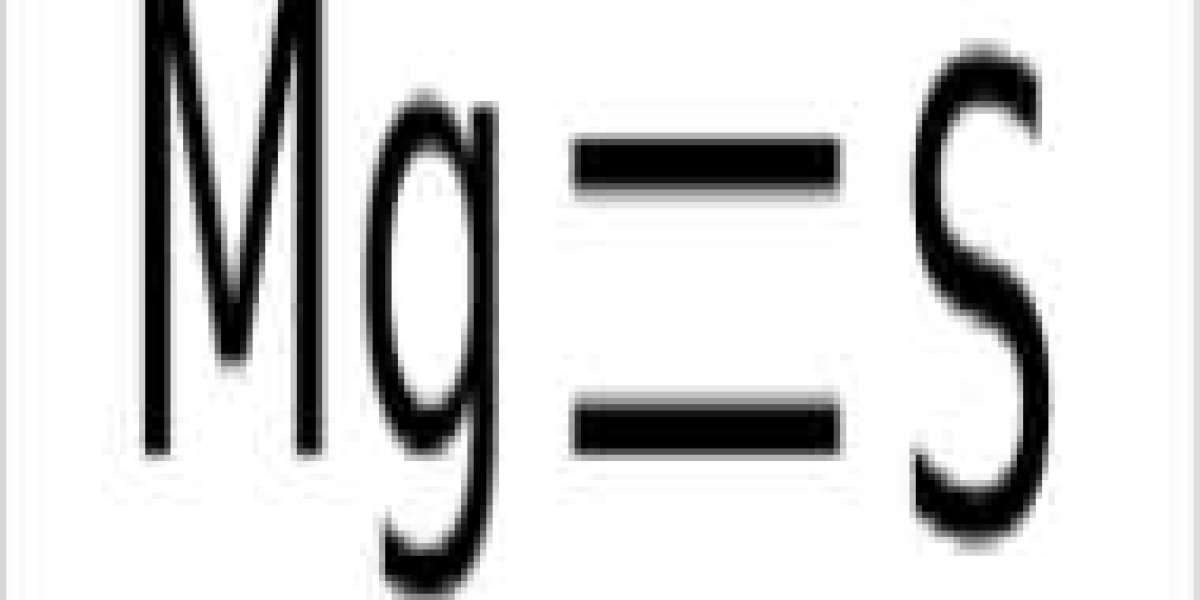Magnesium sulfide is a source of magnesium that is moderately soluble in water and acids and is suitable for sulfate-compatible uses. Sulfate compounds are sulfates or esters formed by substituting a metal for one or two hydrogens. Most metal sulphate compounds are readily soluble in water and are used in applications such as water treatment, unlike fluorides and oxides which are less soluble. Organometallic forms are soluble in organic solutions and sometimes in aqueous and organic solutions. Metal ions can also be dispersed using suspended or coated nanoparticles and deposited using sputtering targets and evaporated materials for applications such as solar materials and fuel cells. Most volumes of magnesium sulphide are usually readily available. High purity, submicron and nanopowder forms are contemplated. American Elements manufactures many standard grades where applicable, including Mil Spec (Military Grade); ACS, Reagent and Technical; Food, Agricultural and Pharmaceutical; Optical, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follow applicable ASTM testing standards. Typical and custom packaging available. Provides additional technical, research and safety (MSDS) information as well as a reference calculator for converting relevant units of measurement.
Magnesium sulfide is brown and crystalline in nature. It is formed when sulfur or hydrogen sulfide reacts with magnesium. It exists in the circumstellar envelopes of some evolved carbon stars and meteorites. Let us understand the chemical composition of magnesium sulfide.
Magnesium sulfide contains a magnesium metal cation charged with Mg+2 and a non-metallic sulfur anion charged with S−2. It has a cubic crystal structure and is white to reddish brown in color.
A -2 sulfide ion is required to balance a +2 magnesium ion, forming magnesium sulfide.