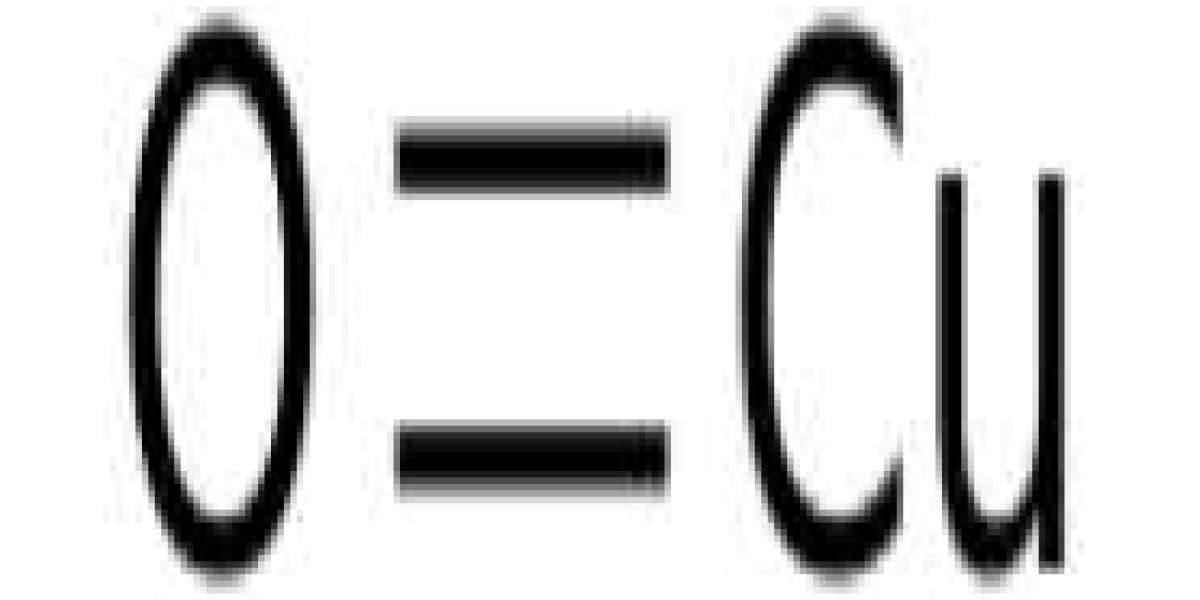Copper(II) oxide, CuO with a monoclinic structure, X-ray photoelectron spectroscopy, and high-resolution transmission electron microscopy studies confirmed the presence of trace amounts of Cu3+ in the annealed CuO. The dielectric behavior of CuO can be explained by a Maxwell-Wagner type polarization mechanism and a thermal activation mechanism.
However, the combination of Au embedded in polyvinyl methylketone film with Cu NPs showed potent antibacterial activity against Gram-negative bacteria. It has been observed that the best antimicrobial activity is achieved through the release of copper ions (Cioffi et al., 2005). Highly ionic CuO NPs with extremely high surface area and unusual crystal morphology serve as an effective fungicide against bacterial pathogens. CuO NPs have high antibacterial activity against Gram-positive bacteria. This is attributed to copper's greater affinity for amine and carboxyl groups present on the cell surface. In addition, the released copper ions become embedded in nucleic acids and intracellular proteins, disrupting biochemical processes.
No peaks characteristic of the copper-containing phase were found in any of the solids examined, thus indicating the very small size of the copper entities.
A 28-40% CuO phase dispersion evaluated by selective N2O reduction at T=90 °C on a Micromeritics AutoChem II 2920 catalyst characterization system confirmed the above statement and revealed copper particle sizes, which were much smaller ( Table 1) Compared to chemically identical materials prepared by co-precipitation, sol-gel, or other traditional methods.