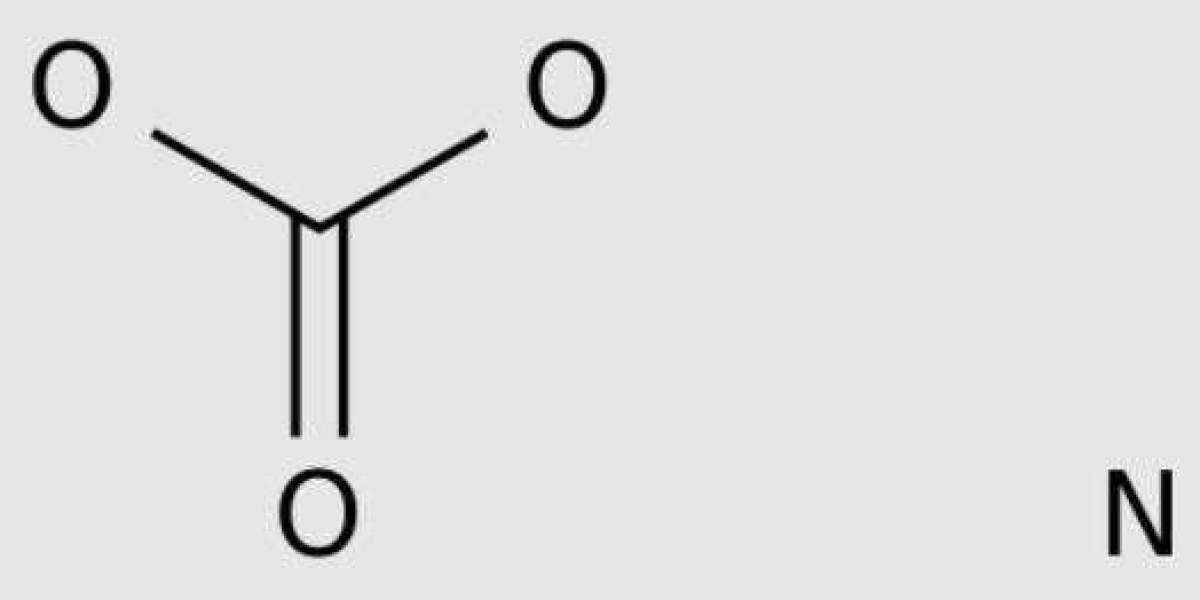
Ammonium bicarbonate is a commonly used inorganic salt in industry. This salt can be formed by combining gaseous carbon dioxide with aqueous ammonia.
Ammonium bicarbonate is used in the food industry to enhance the quality of baked goods such as biscuits and crackers. This material is usually used before adding yeast powder at home.
The History of Ammonium Carbonate
Ammonium bicarbonate was made in the Middle Ages by burning or distilling substances containing creatine (creatine is a structural protein found in the animal kingdom). They achieve this by heating crushed horns, venom, skin, and even mammalian urine in a lime kiln (a kiln used to heat limestone). This is a production process that can be traced back to the Greek and Roman times, converting quicklime into building materials.
After the furnace cools down, the waste is collected. Because wood ash is often boiled in water and used as a paste, these ashes may be used in the same way. It is used to treat fever, various stomach diseases, especially diarrhea, fever, and various bites.
This name was particularly important in the Middle Ages, when people believed that salt collected from burning certain parts of animals had special medicinal value. In the Victorian era, this was a common salt flavor.
Properties of ammonium bicarbonate
Ammonium bicarbonate is a inorganic compound; Chemically, it is an ammonium bicarbonate ion salt. Due to its physical and chemical properties, it is used as a fermentation and stabilizer, as well as an acidity regulator.
The stable properties of ammonium bicarbonate enable it to maintain ideal physical and chemical properties in food. In addition, it can also adjust the pH value by controlling or changing the acidity or alkalinity of food.
Finally, as a kneading preparation, ammonium bicarbonate can be used alone or in combination with other ingredients to increase the dough and volume of baked goods, while also possessing the ability to release gas.
Appearance: Colorless or white crystals with a faint odor of ammonia gas
Melting point: 95 degrees Fahrenheit (35 degrees Celsius)
Solubility: Easily soluble in water. Soluble in ethanol
Decomposition to generate ammonia above 34 ℃
Function
Ammonium bicarbonate can release ammonia and carbon dioxide, causing the cooling gas to react without reacting with dough acid. Firstly, it spontaneously decomposes in the substance and ammonia gas, and in the next stage, it is converted into ammonia and carbon dioxide:
NH4HCO3 → NH4HCO3 + NH3
Application
The use of ammonium bicarbonate is mentioned in ancient cooking recipes. Especially in Scandinavian gingerbread, Polish ammonia biscuits, and traditional German Christmas cookies.
The advantages of using this substance in cooked food include:
No common alkaline flavor residues in sodium bicarbonate
Does not affect the pH value of cooked food
Ammonium bicarbonate may produce an ammonia odor in products cooked at high humidity (over 5%). That's why it's more suitable for low moisture products such as cookies, biscuits, biscuits, and waffles.
Ammonium carbonate is used as a dough in cookies, flat biscuits, or crackers. In German cuisine, it is called hirschhornsalz or harthorn, also known as baker's ammonia. On the other hand, it does not leave behind the salty taste of soap or residue with similar flavors, as it completely decomposes into ammonia and carbon dioxide.