Regulatory Affairs Outsourcing Market Projected to Reach USD 13.56 Billion by 2030
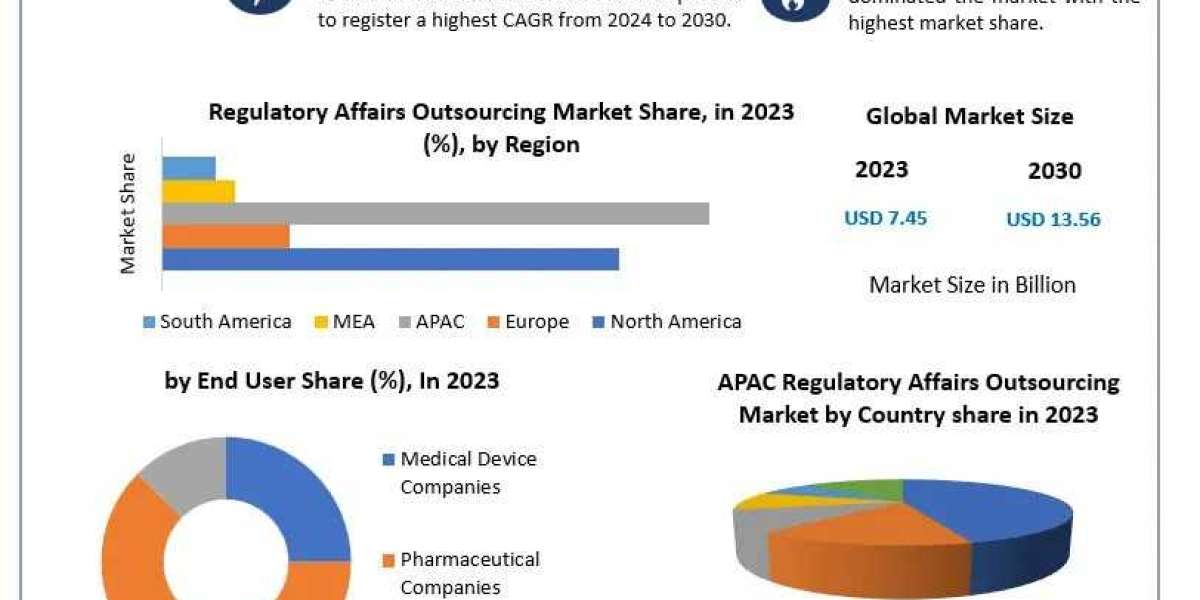
The Regulatory Affairs Outsourcing Market Growth is experiencing significant growth, with projections indicating an increase from USD 7.45 billion in 2023 to approximately USD 13.56 billion by 2030, reflecting a Compound Annual Growth Rate (CAGR) of 8.93%. This expansion is driven by the escalating complexity of regulatory requirements, increased research and development (RD) activities, and the growing need for cost-effective compliance solutions across various industries.
Get To Know More About This Market Study: https://www.maximizemarketresearch.com/request-sample/44332/
Market Definition and Overview
Regulatory affairs outsourcing involves delegating regulatory compliance and documentation tasks to external specialized service providers. This practice is prevalent in highly regulated sectors such as pharmaceuticals, biotechnology, and medical devices. By outsourcing these functions, companies can leverage the expertise of professionals adept in navigating complex regulatory landscapes, ensuring timely approvals, and facilitating market access.
Key Growth Drivers and Opportunities
Several factors are contributing to the robust growth of the regulatory affairs outsourcing market:
Complex Regulatory Landscapes: Industries such as pharmaceuticals and medical devices are subject to stringent and continually evolving regulations. Outsourcing enables companies to stay abreast of these changes without overburdening internal resources.
Globalization of Markets: As companies expand into new geographies, they encounter diverse regulatory frameworks. Outsourcing provides access to regional expertise, ensuring compliance across multiple jurisdictions.
Cost Efficiency: Maintaining in-house regulatory affairs teams can be cost-prohibitive, especially for small and medium-sized enterprises. Outsourcing offers a scalable solution, reducing overhead costs associated with training, technology, and facilities.
Focus on Core Competencies: By outsourcing regulatory functions, companies can concentrate on their primary business activities, such as product development and innovation, thereby enhancing overall productivity.
Technological Advancements: The integration of advanced technologies, including artificial intelligence and machine learning, into regulatory processes has improved efficiency and accuracy. Outsourcing partners often have access to these technologies, providing a competitive advantage.
Request Sample Link For More Details: https://www.maximizemarketresearch.com/request-sample/44332/
Segmentation Analysis
The regulatory affairs outsourcing market is segmented based on service type, company size, category, indication, product stage, end-user, and region:
By Service Type:
- Regulatory Consulting: Offers strategic guidance on regulatory submissions, compliance strategies, and development planning.
- Legal Representation: Provides legal support and representation in interactions with regulatory authorities.
- Regulatory Writing Publishing: Involves the preparation and submission of regulatory documents, including clinical study reports and submission dossiers.
- Product Registration Clinical Trial Applications: Assists in obtaining approvals for new products and managing clinical trial applications.
- Regulatory Submissions: Manages the submission of documents to regulatory bodies to obtain and maintain product approvals.
- Regulatory Operations: Encompasses activities such as regulatory intelligence, compliance audits, and lifecycle management.
- Other Services: Includes training, quality assurance, and risk management services.
By Company Size:
- Small Enterprises: Often lack dedicated regulatory teams and benefit significantly from outsourcing to access specialized expertise.
- Medium Enterprises: May have limited in-house capabilities and outsource to enhance efficiency and manage costs.
- Large Enterprises: Even with established regulatory departments, large companies outsource to handle overflow activities or access regional expertise.
By Category:
- Pharmaceuticals: Involves drugs and therapeutic products requiring stringent regulatory compliance.
- Biologics: Includes products derived from biological sources, such as vaccines and biosimilars, which have complex regulatory pathways.
- Medical Devices: Encompasses a wide range of health-related devices, each subject to specific regulatory standards.
By Indication:
- Oncology: Cancer-related products, a rapidly growing segment due to ongoing research and development.
- Neurology: Products addressing neurological disorders, requiring specialized regulatory knowledge.
- Cardiology: Cardiovascular products, including drugs and devices, with specific regulatory requirements.
- Immunology: Products related to immune system disorders, often involving complex biologics.
- Others: Includes various other therapeutic areas such as endocrinology and gastroenterology.
By Product Stage:
- Preclinical: Early-stage research requiring regulatory support for laboratory and animal studies.
- Clinical: Involves human trials, necessitating comprehensive regulatory submissions and compliance.
- Post Market Authorization (PMA): Ongoing regulatory activities after a product has been approved for market, including compliance monitoring and reporting.
By End-User:
- Pharmaceutical Companies: Engage in drug development and rely on outsourcing for efficient regulatory management.
- Biotechnology Companies: Focus on innovative biological products, often outsourcing due to limited in-house resources.
- Medical Device Companies: Develop a range of health-related devices, outsourcing regulatory tasks to navigate complex approval processes.
Get To Know More About This Market Study: https://www.maximizemarketresearch.com/market-report/global-regulatory-affairs-outsourcing-market/44332/
Regional Insights
North America: Holds a substantial share of the regulatory affairs outsourcing market, driven by a robust pharmaceutical industry and complex regulatory frameworks. The United States, in particular, has a well-established market due to the presence of major industry players and stringent FDA regulations.
Asia Pacific: Expected to witness the fastest growth, attributed to the expansion of pharmaceutical and biotechnology sectors in countries like China and India. The availability of skilled professionals at competitive costs makes this region attractive for outsourcing services.
Europe: Dem
Regulatory Affairs Outsourcing Market Key Players:
Regulatory Affairs Outsourcing Service Providers in North America:
1. Accell Clinical Research, LLC - United States
2. Genpact - United States
3. CRITERIUM, INC - United States
4. Promedica International - United States
5. Medpace - United States
6. Charles River Laboratories - United States
7. Covance, Inc. (Labcorp Drug Development) - United States
8. Parexel International Corporation - United States
9. APCER Life Sciences, Inc. - United States
10. VCLS - United States
11. ProPharma Group MIS Limited - United States
Regulatory Affairs Outsourcing Service Providers in Europe:
1. ICON plc – Ireland
2. PHARMALEX GMBH - Germany
3. NDA Group AB - Sweden
4. Pharmexon - Denmark
5. Qvigilance - United Kingdom
6. BlueReg - France
7. Cambridge Regulatory Services - United Kingdom
8. Regulatory Pharma Net srl - Italy
9. ZEINCRO - Greece
10. BioMapas - Lithuania
11. REGENOLD GMBH – Germany
12. PrimeVigilance - United Kingdom
13. Real Regulatory Ltd - United Kingdom
Regulatory Affairs Outsourcing Service Providers in Asia Pacific:
1. WuXi AppTec – China
2. Freyr - India
??? ?????????? ????????, ?????:
Surgical Imaging Market https://www.maximizemarketresearch.com/market-report/global-surgical-imaging-market/31709/
Global Infrared Heat Lamp Market https://www.maximizemarketresearch.com/market-report/global-infrared-heat-lamp-market/87424/
Compliance and Traceability Solutions Market https://www.maximizemarketresearch.com/market-report/compliance-and-traceability-solutions-market/168369/
Surgical Rasps Market https://www.maximizemarketresearch.com/market-report/surgical-rasps-market/216028/
About Maximize Market Research:
Maximize Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Maximize Market Research:
MAXIMIZE MARKET RESEARCH PVT. LTD.
⮝ 3rd Floor, Navale IT Park Phase 2,
Pune Banglore Highway, Narhe
Pune, Maharashtra 411041, India.
✆ +91 9607365656
? sales@maximizemarketresearch.com
? www.maximizemarketresearch.com