Neurofibromatosis type 1-associated plexiform neurofibromas (NF1-PN) remain one of the most challenging manifestations of NF1, characterized by the growth of complex nerve sheath tumors in areas that are often anatomically challenging. Historically, the management of NF1-PN has been dominated by surgical interventions, yet the complexity of these tumors—especially when located near vital structures—often limits the effectiveness of surgery. In recent years, however, breakthroughs in targeted therapies and emerging market insights have signaled a transformative shift in NF1-PN treatment. This article provides an in-depth exploration of the evolving landscape, examining NF1-PN epidemiology, market trends, innovative therapies, and future growth forecasts.
Understanding NF1-PN Epidemiology and NF1-PN Market Trends
NF1-PN is rooted in genetic mutations that lead to the abnormal proliferation of nerve tissue. Although these tumors are usually benign, their size and location can cause significant morbidity, affecting vital functions and leading to a diminished quality of life. Epidemiological data reveal that NF1-PN is not only clinically challenging but also presents a substantial market opportunity. In the United States, NF1-PN accounts for a significant share of the oncology market, with net sales revenue reaching nearly USD 230 million in 2023. Meanwhile, in Europe—particularly in Germany—market estimates approach USD 32 million, underscoring robust NF1-PN Market Size and dynamic NF1-PN Market Trends across key regions including the United States, EU4, the United Kingdom, and Japan.
A closer look at patient demographics further enhances our understanding of the condition. Approximately 30% of NF1-PN cases in the United States occur in pediatric populations, with the remaining 70% diagnosed in adults. This age distribution emphasizes the necessity for tailored therapeutic strategies that cater to both younger patients and adult populations. By leveraging comprehensive NF1-PN Epidemiology data, stakeholders are better equipped to develop market strategies and allocate resources effectively to meet patient needs.
For more in-depth insights on NF1-PN treatments and challenges, download the full report @ NF1-PN Market Report.
The Challenges of Traditional NF1-PN Treatments and the Shift to NF1-PN Therapies
Historically, surgical resection has been the primary treatment for NF1-PN. However, the intricacies of the tumors often make surgery a risky proposition. The potential for surgical complications, along with the high likelihood of tumor recurrence, has prompted clinicians to seek alternative therapies. Surgical interventions, while sometimes necessary, can result in significant morbidity due to the delicate locations of these tumors. The limitations of traditional surgery have paved the way for the development of medical therapies that target the underlying genetic mechanisms driving NF1-PN.
As the focus shifts toward pharmacological treatments, innovative NF1-PN Therapies have begun to gain prominence. Advances in genetic research have catalyzed the development of targeted therapies that offer greater precision with fewer side effects. This paradigm shift in treatment not only holds the promise of improved clinical outcomes but also aligns with the broader movement toward personalized medicine in oncology.
Advancements in NF1-PN Drugs and the Expanding NF1-PN Pipeline
Among the most promising developments in the field of NF1-PN treatment is the emergence of targeted therapies, particularly MEK inhibitors. KOSELUGO—a MEK inhibitor developed through the collaborative efforts of AstraZeneca and Merck—has emerged as a significant breakthrough. Its mechanism of action disrupts the cellular signaling pathways responsible for tumor growth, presenting a viable alternative for patients, especially pediatric cases where surgery is not feasible.
The NF1-PN Pipeline is further enriched by a host of emerging drugs. Mirdametinib from SpringWorks Therapeutics and FCN-159 from Fosun Pharmaceutical are currently undergoing clinical evaluation, showcasing encouraging preliminary results. PAS-004, developed by Pasithea Therapeutics, has advanced through Phase 1 clinical trials without encountering dose-limiting toxicities. Early indications suggest that patients might benefit from higher dosing regimens, as external safety review committees have endorsed continuing higher doses without modification. Additionally, HLX-1502 from Healx received Fast Track Designation from the US FDA in October 2024, a milestone that not only accelerates its development but also highlights its potential to address significant unmet needs in NF1-PN treatment.
These developments underscore the critical role that NF1-PN Companies are playing in redefining treatment paradigms. By investing heavily in research and development, these companies are expanding the NF1-PN Pipeline and accelerating the availability of next-generation therapies. Their contributions are essential in driving innovation, reducing treatment-related complications, and ultimately improving the quality of life for patients.
For more detailed insights and the latest updates on NF1-PN Pipeline drugs visit the NF1-PN Emerging Drugs.
NF1-PN Forecast and Market Growth Prospects
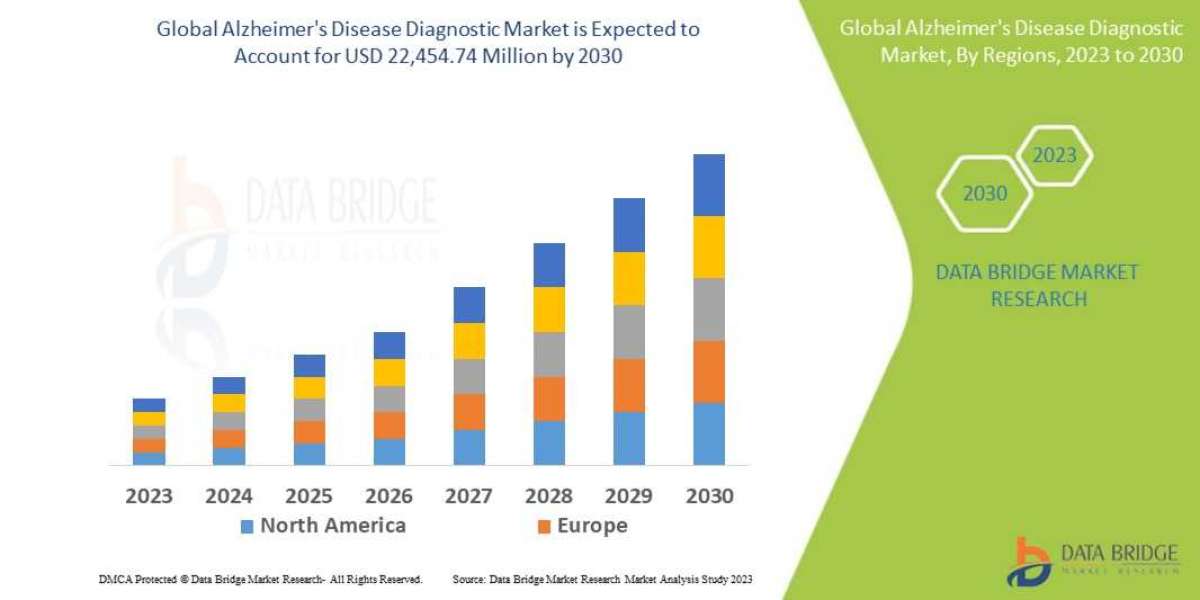
Market forecasts suggest that the NF1-PN landscape is poised for substantial growth in the coming decade. With the projected net sales revenue estimated at approximately USD 380 million in 2023, the market is expected to experience significant upward momentum through 2034. This growth is fueled not only by the ongoing development of novel drugs but also by the increasing prevalence of NF1-PN and enhanced awareness among healthcare professionals and patients alike.
The integration of advanced diagnostic tools and a deeper understanding of NF1-PN’s molecular underpinnings is set to revolutionize patient management. Early intervention and comprehensive care strategies—supported by a multidisciplinary approach—are likely to improve outcomes while reducing the burden of the disease. As these trends continue to evolve, the NF1-PN Market Forecast signals a period of robust expansion, driven by both innovation in therapeutic modalities and strategic investments in healthcare infrastructure.
The Role of NF1-PN Companies in Driving Innovation
A significant catalyst behind the evolving NF1-PN treatment landscape is the concerted effort of leading pharmaceutical and biotechnology companies. Major players such as AstraZeneca, Merck, SpringWorks Therapeutics, Fosun Pharmaceutical, Pasithea Therapeutics, and Healx are spearheading research initiatives that are redefining the therapeutic approach to NF1-PN. Their groundbreaking work in developing targeted therapies not only addresses the limitations of conventional surgery but also sets new benchmarks in precision medicine.
These NF1-PN Companies are instrumental in advancing clinical research, facilitating faster regulatory approvals, and fostering collaborative networks that span across disciplines. The result is a vibrant, dynamic market characterized by a steady influx of innovative drugs and a commitment to improving patient care. As these companies continue to push the boundaries of what is possible, they are simultaneously shaping market trends and ensuring that the treatment landscape remains responsive to the evolving needs of patients and clinicians.
Embracing a Multidisciplinary Approach for Comprehensive NF1-PN Care
Effective management of NF1-PN requires the concerted efforts of a multidisciplinary team that includes geneticists, neurologists, radiologists, and surgeons. By working collaboratively, these specialists can develop personalized treatment plans that address both the physical and emotional challenges posed by the condition. This integrated approach is critical for managing the complex interplay between tumor growth, symptom management, and overall patient quality of life.
A multidisciplinary strategy not only enhances the precision of treatment but also facilitates the integration of emerging NF1-PN Therapies into standard care protocols. With comprehensive care plans that encompass both surgical and pharmacological interventions, healthcare providers can better navigate the challenges of NF1-PN. This collaborative model is essential for delivering holistic patient care and is a key driver of improved clinical outcomes in a landscape that is rapidly evolving.
For further insights and detailed research on NF1-PN Epidemiology, visit the NF1-PN patient pool.
Future Perspectives and Conclusion
The future of NF1-PN treatment is marked by continuous innovation, driven by an increasing understanding of the genetic and molecular mechanisms underlying the disease. The transition from a sole reliance on surgical intervention to the adoption of targeted pharmacotherapies signifies a transformative moment in NF1-PN care. With an expanding NF1-PN Pipeline, significant market growth forecasts, and robust support from leading NF1-PN Companies, the horizon for patients afflicted by this challenging condition is filled with promise.
The convergence of research, clinical expertise, and market dynamics is creating a fertile environment for breakthrough innovations that are set to redefine NF1-PN treatment paradigms. As new therapies such as KOSELUGO, Mirdametinib, FCN-159, PAS-004, and HLX-1502 become integral to treatment protocols, patients can look forward to options that offer enhanced safety, efficacy, and improved quality of life. Moreover, the adoption of a multidisciplinary approach ensures that treatment strategies are comprehensive and patient-centric, further bolstering the prospects for successful management.
In summary, the emerging innovations in the treatment of Neurofibromatosis type 1-associated plexiform neurofibromas are not only transforming the clinical landscape but also heralding a new era of personalized medicine. The robust NF1-PN Market, supported by evolving epidemiological insights and market trends, is poised for significant growth, offering renewed hope to patients and clinicians alike. As the journey toward improved management and care continues, the collaborative efforts of NF1-PN Companies, researchers, and healthcare professionals will remain pivotal in overcoming the challenges of this complex condition.
The advances made in recent years underscore a commitment to redefining the treatment landscape for NF1-PN—a commitment that is set to yield substantial clinical benefits and market opportunities in the years ahead. With an unwavering focus on innovation and patient care, the future of NF1-PN treatment is bright, promising a more precise, effective, and compassionate approach to managing one of the most challenging aspects of Neurofibromatosis type 1.
To understand which factors are driving NF1-PN market trends, download our full report.
Read More
- Plexiform neurofibroma Pipeline Insight
- Neurofibromatosis Type 1 (NF1) Epidemiology
- Cutaneous Lupus Erythematosus Market Insight
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.