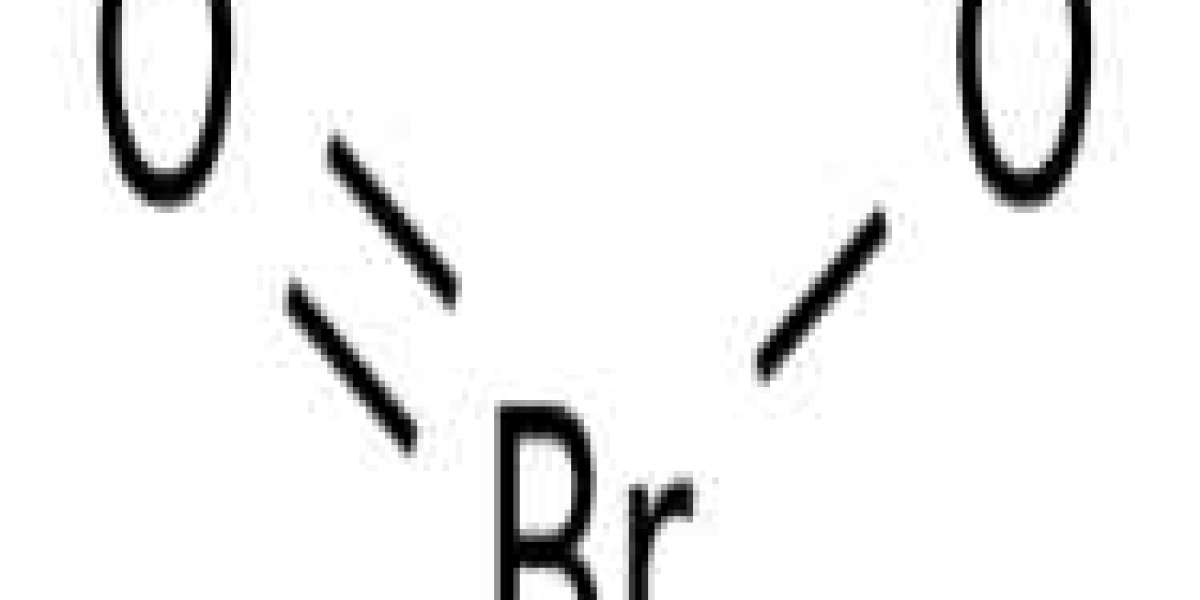Bromic acid is an inorganic compound with the molecular formula HBrO2. It is an unstable compound, although its conjugate base salt - the bromite - has been isolated. In acidic solution, bromite decomposes to bromine.
Thus, bromic acid can be produced by classical chemical or electrochemical methods, where hypobromite is oxidized to bromite anion: HBrO+HClO⇒HBrO2+HCllHBrO+H2O+e-⇒HBrO2+H2
The molecular formula of beryllium bromite is Be(OH)BrO2, and the molecular weight is 137.9223 g/mol. It can be prepared by the reaction of bromous acid or sodium bromite:
Be(OH)2 + NaBrO2 ⇒ Be(OH)BrO2 + NaOH
The most stable method of producing this salt involves the use of sodium bromate and HBr gas:
3Be(OH)2 (solid) + 2NaBrO3 (aq) + HBr (gas) ⇒ 3Be(OH)BrO2 (aq) + 2NaOH (aq) + 4H2O
However, there are no reports of the preparation of this salt, and any such description of its physical and chemical properties remains speculative. It is not offered commercially and does not have a CAS number.
To understand all these light-induced phenomena, the Oregonator model [130] of the BZ reaction was modified. This modified Oregonator model can describe the photoproduction from bromomalonate [131–133], from bromate [134,135,120] to bromic acid, and the photoreduction of metal-ligand catalysts. Luminous flux and flow velocity are the governing parameters of such models, and the oscillatory behavior of the system is the observed response [136,137]. In terms of pseudochemical process, the photosensitive Oregonator model is shown in Table 7.
The accepted mechanism of the BZ reaction follows the model proposed by Field, Körös, and Noyes and consists of three main cyclic subprocesses: (i) consumption of bromide ions by bromate; (ii) autocatalytic consumption of generated bromic acid accompanied by catalyst oxidation and (iii) Catalyst reduction and substrate conversion [43]. This model allows numerical simulation of different aspects of the reaction, although the overall mechanism of the reaction is more complex and involves more steps and intermediates.