Learn the chemical formulas of various other compounds here.
Preparation of boric acid
One of the easiest ways to prepare boric acid is to react borax with any mineral acid such as hydrochloric acid. The chemical equation for this reaction can be written as:
Na2B4O7.10H2O + 2HCl → 4H3BO3 + 5H2O + 2NaCl
Boronic acid can also be prepared by hydrolysis of diborane and boron trihalides (such as boron trichloride or boron trifluoride).
Properties of Boronic Acid – H3BO3
Under standard temperature and pressure (STP) conditions, boric acid exists as a white crystalline solid that is very soluble in water. The solubility of H3BO3 in water is temperature dependent. The solubility of boric acid in water at 25 °C is 57 g/L. However, when water is heated to 100 °C, the solubility of this compound increases to approximately 275 g/L. It can also be noted that boric acid is slightly soluble in pyridine and slightly soluble in acetone. The conjugate base of boric acid is the borate anion.
The acidity of boric acid solutions is known to increase with polyols containing cis-vicinal diols such as mannitol and glycerol. The pK of B(OH)3 is known to extend over five orders of magnitude (from 9 to 4) at different concentrations of mannitol. It may be noted that in the presence of mannitol, a boric acid solution with increased acidity may be referred to as mannose boronic acid.
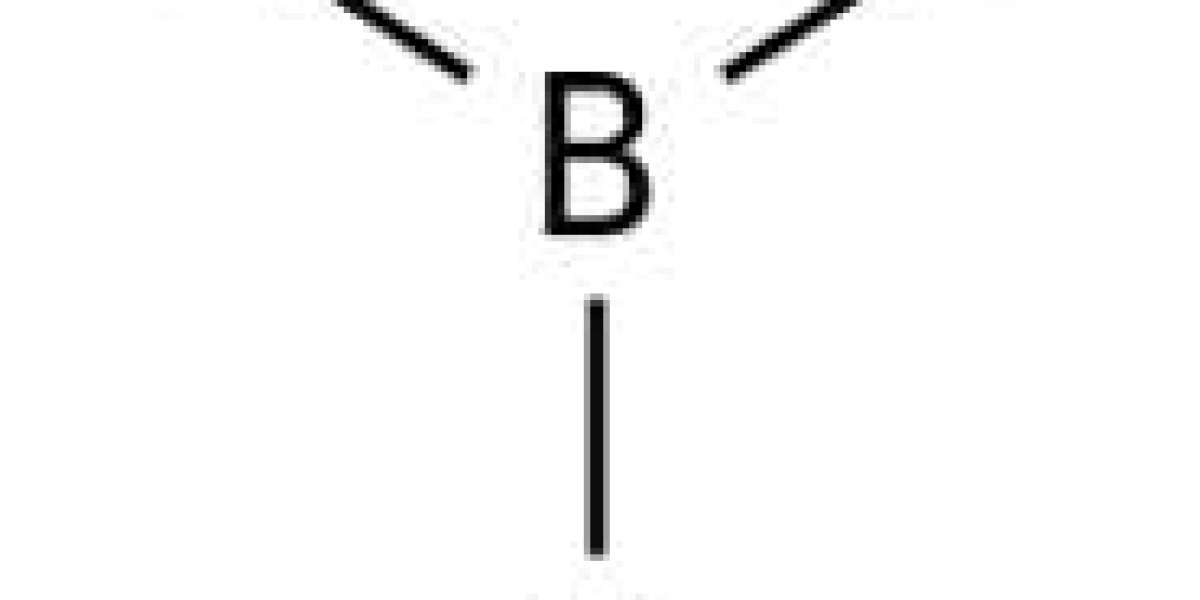
The structure of the H3BO3 molecule
Each boronic acid molecule has a boron-oxygen single bond. A boron atom occupies the central position and is connected to three hydroxide ions. The overall molecular geometry of boronic acid is triangular planar. The structure of the H3BO3 molecule is shown in the figure below.