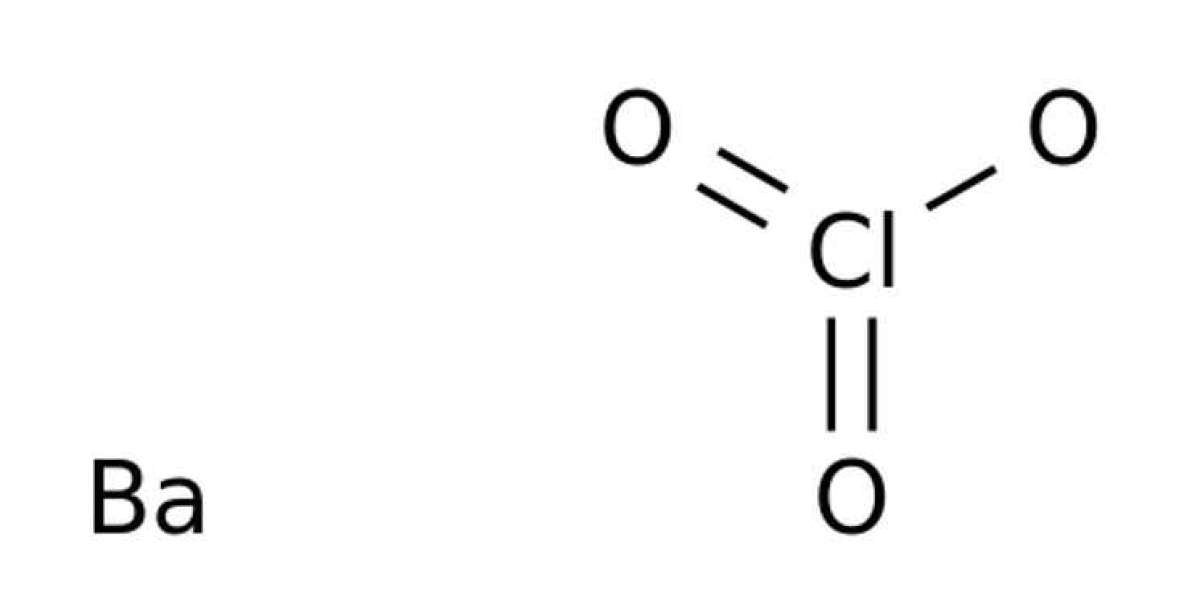
Some notes on barium chlorate chemistry
The ability of a molecule to act as an oxidizer is at best only vaguely related to the count of oxygens per molecule.
Barium chlorate has a formula weight of 304.23. Sure, there are 6 oxygens, but there is a lot of weight in the Barium atoms. A 100 gram sample of barium chlorate contains 31.55 grams of oxygen atoms. Potassium chlorate has a formula weight of 122.55, with 3 oxygens. This means that a 100 gram sample contains 39.17% oxygen. In fact, on a weight/weight basis, there is more oxygen in a gram of Potassium Chlorate than in a gram of barium chlorate.
Next - Barium surprisingly has essentially the same radius as Potassium. According to CRC, Crystal Ionic Radii, 59'th Ed, page F-213, the radii of Barium 2+ and Potassium + are 1.34 Ang, and 1.33 Ang respectively. (Why? There are two charges on the Ba, which means that the counterion is more strongly attracted, and quantum mechanical theory shows that when low lying d-orbitals are available there may be a surprising amount of electron density and back bonding in those orbitals, thus creating an anisotropic opportunity for unusually close approach). So, the difference in the crystal lattice stability cannot be ascribed to the difference in size.
Then we get to the greater conundrum heats of formation. Barium chlorate is actually more stable than Potassium Chlorate, as regards formation from the elements. The heat of formation of Ba(ClO3)2 is -184.4 kcal/mol, while Potassium Chlorate is only -95.1 kcal/mol. However, the heat of decomposition as measured to stable products is much better for Ba(ClO3)2 at -28 kcal/mol, while it is only -10.6 kcal/mole for KClO3. How can this be? It's due to the fact that the products of decomposition are much more stable in the Barium case than in the Potassium case. This means that more heat is released when barium chlorate decomposes. Thus, a small initial reaction returns more heat to the surrounding composition, thus more easily creating a runaway reaction.
Ba(ClO3)2 by electrolyses
Barium chlorate is used for making vivid green colour in pyrotechnics. If you make it by double decomposition with Barium chloride and Sodium chlorate it will be difficult to remove all of the sodium ion from the product and the yellow colour that sodium gives will effect the vivid green colour that you desired. Some have suggested using Calcium chlorate instead of Sodium chlorate.
It can be produced easily enough by electrolyses but the current efficiency is lower than with other chlorates.
The solubilities of the chlorate and the chloride are not very far apart and it is not possible to get a large crop of pure crystals out of the electrolytic cell. This is not a problem as you can take a small crop and recycle the rest. About 40g chloride per 100ml water is put into the cell and the cell is run for the required run time. It should be noted that the chloride has water of crystallization and has the formula BaCl2:2H2O. The current efficiency will be about 30 - 40% without pH control. The cell liquor is evaporated until crystals form and a crop of Ba chlorate removed.
Non-Electrolytic production of barium chlorate
The method for making very pure barium chlorate on a laboratory scale involves an ammonium chlorate stage. KClO3 and (NH4)2 SO4 in solution are evaporated until a thin slurry of K2SO4 begins to form the rest of the K2SO4 is precipitated with alcohol. A solution of NH3ClO3 is left in solution. The alcohol is then removed by distillation (presumably around 70degC) The text then warns that NH4ClO3 has a tendency to explode but neglects to mention whether this is the salt or solution. A barium hydroxide solution is then added and the solution is then kept hot until the ammonia odour has disappears. The solution is then evaporated until dry then redissolved in water. CO2 is bubbled through to remove any barium hydroxide as barium carbonate. The filtrate is then evaporated to crystallisation.