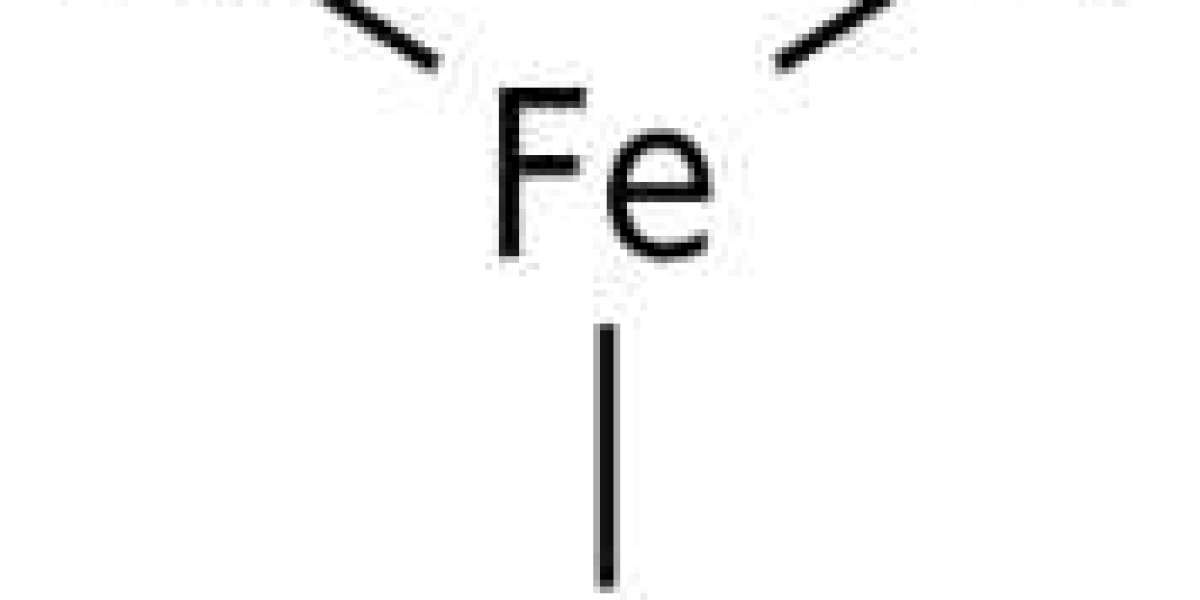
How to find the molar mass of FeCl3.
FeCl3 is iron(III) chloride. The Roman numeral III indicates that this is an Fe+3 ion. Since the chloride ion has a -1 charge, three are needed to cancel the +3 charge of the iron ion.
The molar mass of iron(III) chloride is 162.204 g/mol.
To find the molar mass of FeCl3, we multiply the molar mass of chlorine by three and add that value to the molar mass of iron.
Ferric chloride is an orange to brown-black solid. It is slightly soluble in water. It is nonflammable. When wet, it will corrode aluminum and most metals. Pick up and remove spilled solids before adding water. It is used to treat sewage, industrial waste, purify water, as an etchant for engraving circuit boards, and to make other chemicals.
Ferric chloride solution is a colorless to light brown aqueous solution with a faint smell of hydrochloric acid. Highly corrosive to most metals and may corrode tissue. Non-flammable. For sewage treatment and water purification.
Ferric chloride is an orange to brown-black solid. It is slightly soluble in water. It is nonflammable. When wet, it will corrode aluminum and most metals. Pick up and remove spilled solids before adding water. It is used to treat sewage, industrial waste, purify water, as an etchant for engraving circuit boards, and to make other chemicals.
In liquid state, ferric chloride is an orange-brown aqueous solution with a faint hydrochloric acid smell. It is highly corrosive to metals and organic tissues.
The main application is water treatment. The product is particularly effective in purifying drinking water, purifying wastewater by "thickening" the sediment (ferric chloride is added with lime before the filter press) and dephosphorizing wastewater.