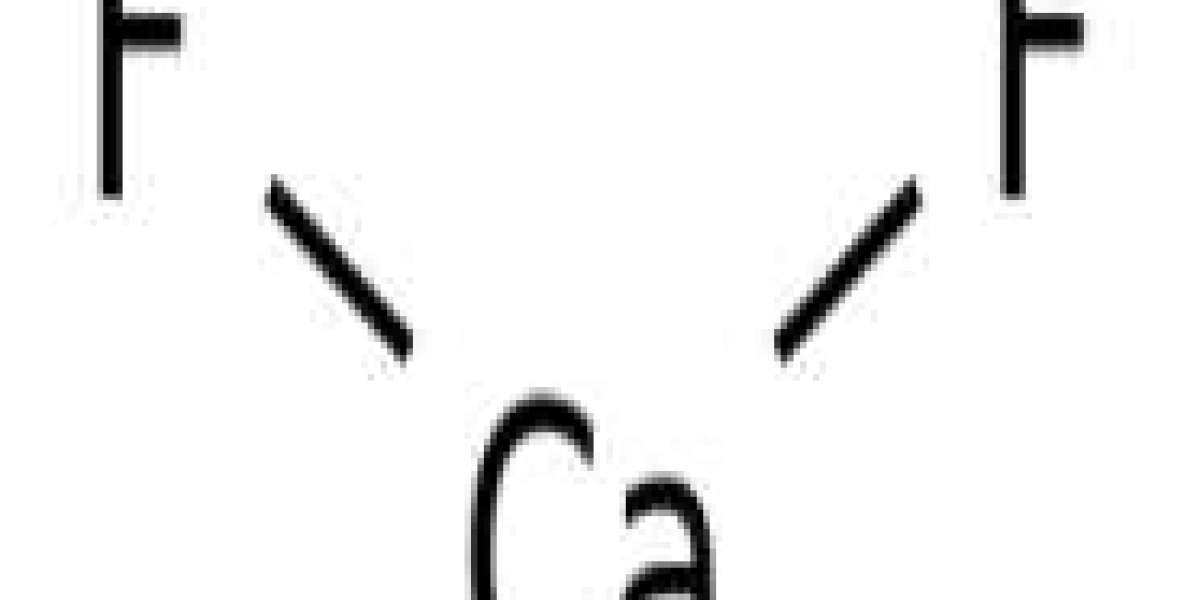
Ca2+ centers are octacoordinates, centered on a cube of eight F- centers. Each F- center coordinates with four Ca2+ centers of tetrahedral shape. [5] Although perfectly packed crystal samples are colorless, minerals are often dark in color due to the presence of F centers. The same crystal structure is found in many ionic compounds of formula AB2, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2. In the corresponding inverse structure, called the inverse fluorite structure, the anions and cations are exchanged, such as Be2C.
Calcium fluoride is an inorganic compound of the elements calcium and fluorine with the chemical formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorspar (also known as fluorspar), and is often dark in color due to impurities.
Instead, they form a calcium fluoride-like substance that deposits on the tooth surface and dissolves when the local pH decreases.
Calcium fluoride (CaF2) is an insoluble ionic compound composed of Ca2+ and F- ions. It occurs naturally in the minerals "fluorite" (also known as fluorite) and "blue john". This salt is the source of most of the world's fluorine.
Synthesis and Characterization of Calcium Fluoride (CaF2)
Calcium fluoride (CaF2) nanoparticles with the ability to release fluoride were prepared by spray drying. The method is summarized below.
Calcium fluoride (CaF2) preparations are of great interest in preventive dentistry because they act as unstable fluoride reservoirs in caries prevention. Low concentrations of fluoride in the range of 0.1 ppm F− in oral fluids from tooth powders or mouthwashes have been shown to have a profound effect on the development of dental caries. However, low salivary calcium concentrations provide a limited driving force for the formation of CaF2 deposits. Nano-CaF2 powders containing 10–15 nm sized crystallite clusters were prepared from Ca(OH)2 and NH4F solutions using a spray-drying technique.