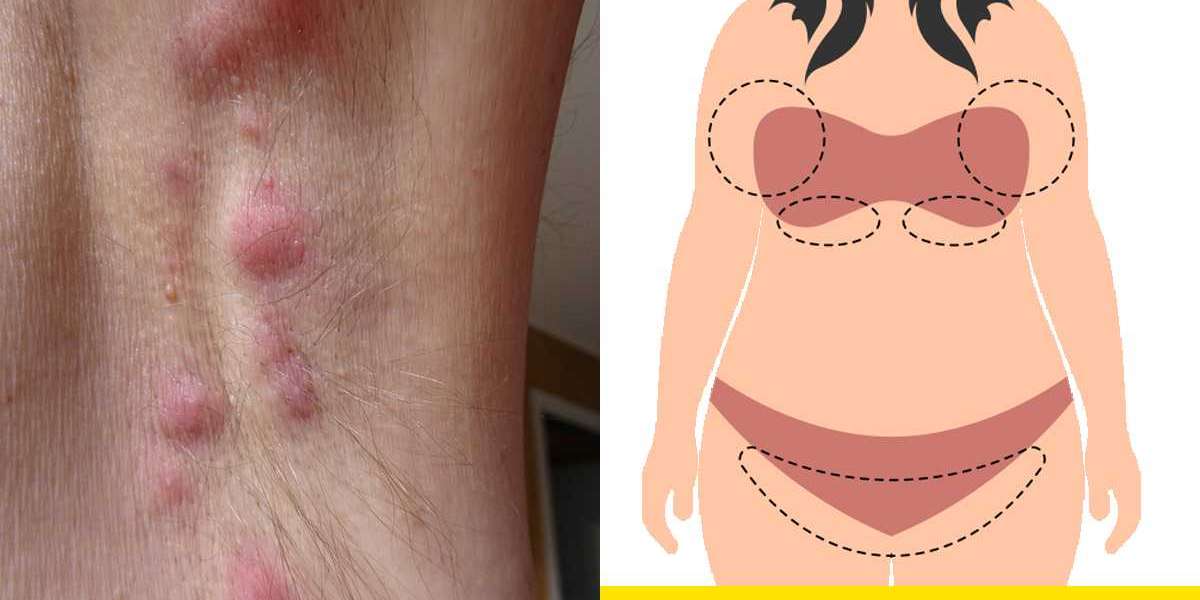
The hidradenitis suppurativa (HS) market is poised for significant transformation, driven by breakthroughs in therapeutic research, epidemiological insights, and a sharpened focus on the unmet needs of patients. Hidradenitis suppurativa, a chronic and debilitating inflammatory skin disease, is characterized by recurrent painful nodules, abscesses, and subsequent scarring, mainly in intertriginous regions. The chronicity and complexity of HS have historically complicated its diagnosis and management, often leaving patients with inadequate treatment options and a diminished quality of life. However, a convergence of factors—ranging from enhanced disease awareness to the approval of innovative biologic therapies—is reshaping the landscape for both patients and the pharmaceutical industry. This article explores the evolving market dynamics, epidemiological trends, competitive landscape, ongoing clinical trials, and future directions for hidradenitis suppurativa, while underscoring the persistent challenges and opportunities ahead.
For insights into the emerging trends and market dynamics shaping the future of Hidradenitis Suppurativa care, explore our in-depth analysis of Hidradenitis Suppurativa treatment market insights.
Hidradenitis Suppurativa Market Overview
The global hidradenitis suppurativa market is set for robust growth, with recent market reports forecasting an impressive compound annual growth rate over the next decade. This trend is propelled by increasing disease awareness among both clinicians and patients, advancements in diagnostic methodologies, and, most notably, the introduction of novel biologics that target key inflammatory mediators associated with hidradenitis suppurativa pathogenesis. The market landscape has evolved rapidly, with established biologics like adalimumab (the first FDA-approved treatment for moderate-to-severe HS) being joined by newer agents such as secukinumab and bimekizumab—the latter recently approved in Europe and currently under review by the US FDA.
Investments in HS research and development have surged, reflecting the substantial market opportunity in this underserved therapeutic area. Major pharmaceutical companies are intensifying their focus on HS, recognizing its significant burden and the demand for more effective treatment options. Innovations in biologic therapies have marked a paradigm shift, expanding beyond the traditional reliance on antibiotics and surgical interventions, and now targeting the underlying inflammatory pathways that drive disease progression and recurrence.
Hidradenitis Suppurativa Epidemiological Trends
An in-depth understanding of hidradenitis suppurativa epidemiology reveals notable regional and demographic variations, which play a critical role in market evaluations and strategy. Western Europe, Scandinavia, the United States, and Australia provide the majority of HS prevalence data, with these regions recording relatively higher rates of the disease. Interestingly, epidemiological patterns highlight a striking gender disparity: HS is more prevalent in females in North America and Europe, whereas countries like South Korea report a male predominance.
The onset of HS typically occurs in late adolescence to early adulthood, with the highest incidence among young adults. Age demonstrates a negative association with disease prevalence, suggesting a potential decline in HS rates with increasing age. Despite advances in awareness campaigns and diagnostic tools, a significant gap persists in epidemiological data from non-Western regions, creating challenges for global market expansion and patient identification strategies.
For further insights and detailed research on Hidradenitis Suppurativa epidemiology, visit the Hidradenitis Suppurativa patient pool.
Recent Developments, Clinical Trials, and Competitive Landscape
The competitive landscape of the hidradenitis suppurativa market is marked by a dynamic and diverse pipeline, featuring candidates with novel mechanisms of action. The approval of biologic agents such as adalimumab (an anti-TNF therapy) and secukinumab (an anti-IL-17A agent) has set the stage for a new era in HS treatment. Bimekizumab, which targets both IL-17A and IL-17F, is a recent addition, offering promise for enhanced efficacy and extended indications.
Pipeline innovation is robust, with clinical trials investigating a range of new agents, including JAK inhibitors like povorcitinib, which could potentially become the first oral therapy approved for HS. Nanobodies such as sonelokimab are also under evaluation, leveraging enhanced tissue penetration to potentially achieve superior clinical outcomes. Beyond these, investigational therapies targeting IL-36, IL-1, and CXCR1/CXCR2 pathways could address the multifaceted pathophysiology of HS. Topical therapies, such as ruxolitinib cream for mild disease, are being explored to fill significant gaps in early-stage HS management.
Leading pharmaceutical companies, including AbbVie, Novartis, UCB, and Incyte, are actively engaged in late-stage clinical trials, seeking to expand both their therapeutic portfolios and the overall scope of the HS treatment market. The expansion of the competitive landscape, supported by robust clinical trial activity, underscores the pharmaceutical industry’s commitment to addressing the substantial unmet needs within the HS patient community.
Market Barriers and Unmet Needs
Despite notable progress, the hidradenitis suppurativa market remains encumbered by several significant barriers. Delayed diagnosis is a pervasive issue, with many patients enduring symptoms for years before accurate identification of their condition. Even with the advent of biologics and improved clinical guidelines, symptom control remains suboptimal for a considerable proportion of patients, with pain, suppuration, and psychological effects contributing to reduced quality of life.
Another critical challenge is the multifactorial nature of HS, which often necessitates multidisciplinary care addressing not only cutaneous symptoms but also metabolic, psychological, and systemic comorbidities. The lack of approved therapies for mild disease and reliance on systemic agents for moderate-to-severe cases perpetuate the need for novel, well-tolerated, and effective treatments across the entire disease spectrum.
For detailed insights on emerging therapies and trends within the Hidradenitis Suppurativa treatment market, download the full report.
Market Drivers and Future Directions
Several interrelated forces are propelling the growth and innovation seen in the hidradenitis suppurativa market. Increased disease awareness—bolstered by patient advocacy organizations, educational campaigns, and continuing medical education for clinicians—has improved diagnosis rates and patient access to advanced therapies. The validation of immune-mediated pathways as therapeutic targets has opened the door for personalized medicine approaches, allowing for the tailoring of treatments based on individual patient profiles and biomarkers.
Future market dynamics will likely be shaped by the continued expansion of the therapeutic pipeline, advances in biomarker discovery, and integration of digital health strategies for patient monitoring and early intervention. The move toward multidisciplinary care models, addressing both physical and psychological aspects of the disease, is expected to improve patient outcomes and expand market opportunities.
Conclusion
In summary, the hidradenitis suppurativa market is undergoing a period of remarkable change, driven by scientific innovation, shifting epidemiological patterns, and an intensified focus on patient-centered care. While biologic approvals and a burgeoning clinical pipeline have substantially expanded treatment options, significant challenges—including delayed diagnosis, persistent unmet needs, and gaps in mild disease management—remain. The future of the hidradenitis suppurativa market will hinge on ongoing collaboration between researchers, clinicians, pharmaceutical companies, and patient communities to refine therapeutic approaches and reduce disease burden. As the global healthcare landscape recognizes the profound impact of HS, the stage is set for transformative advances that promise renewed hope and improved outcomes for patients worldwide.
For further insights and detailed updates on this evolving field, visit our comprehensive insights and expert analysis.
Read More
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.